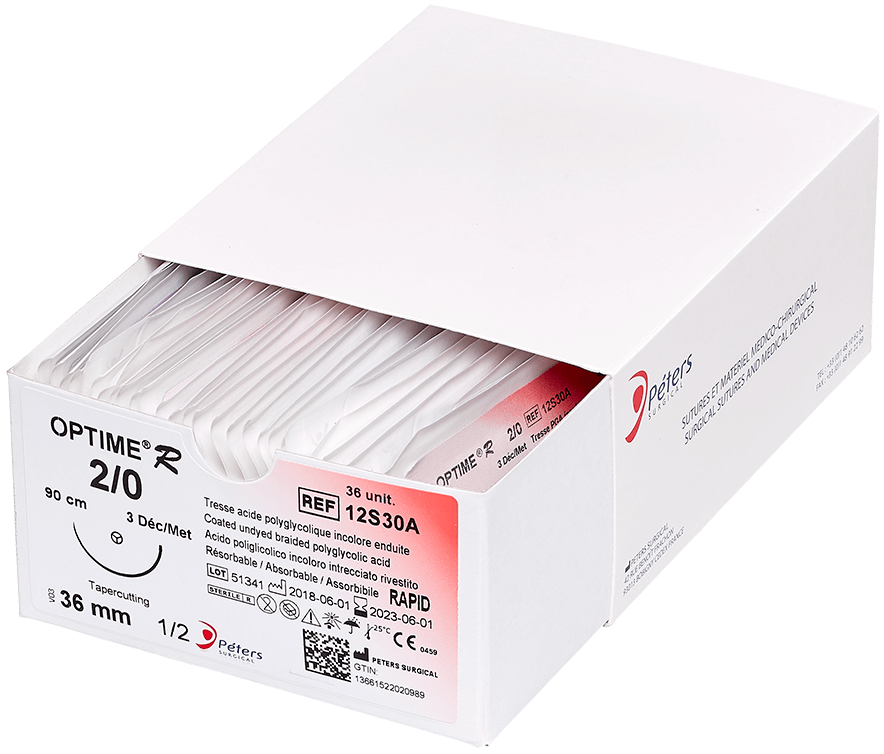
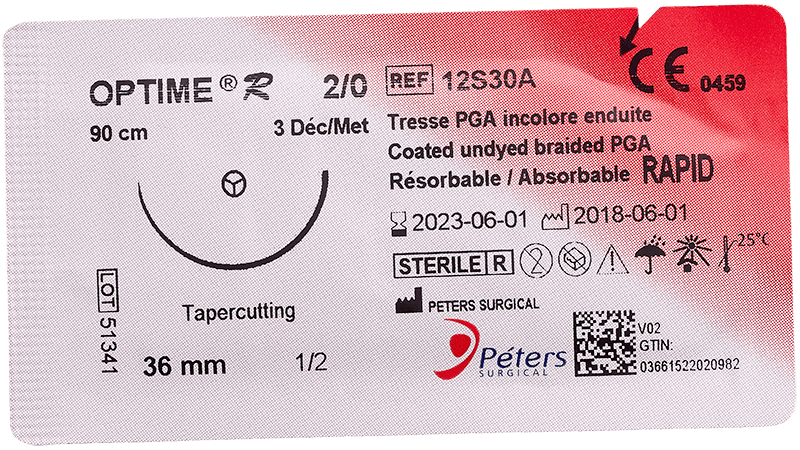
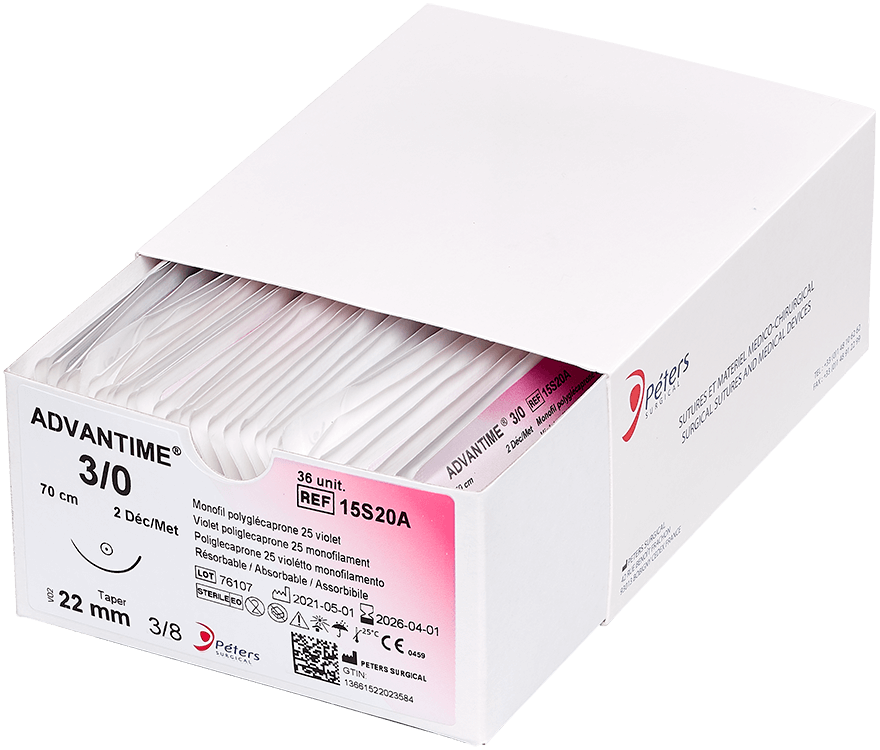
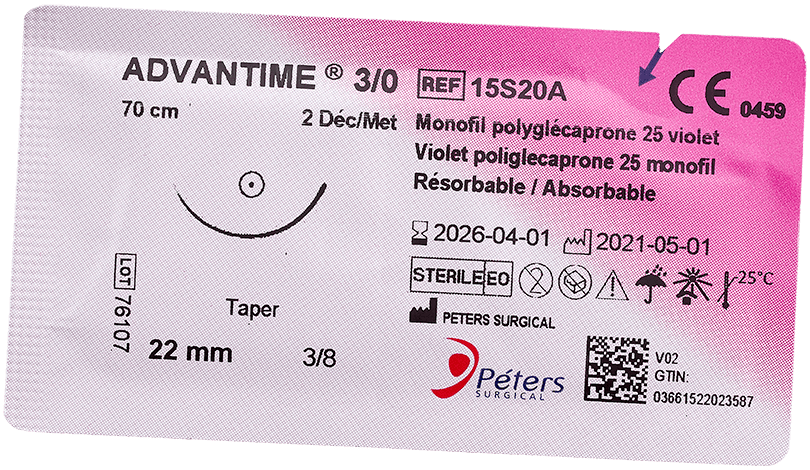
Main advantage of absorbable sutures
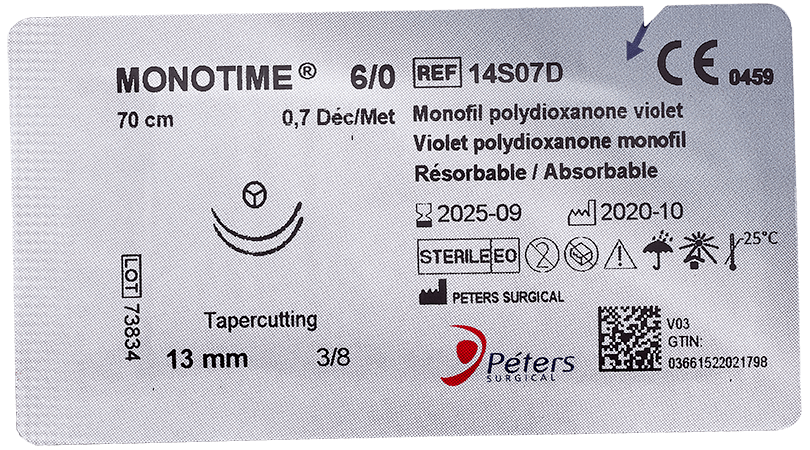
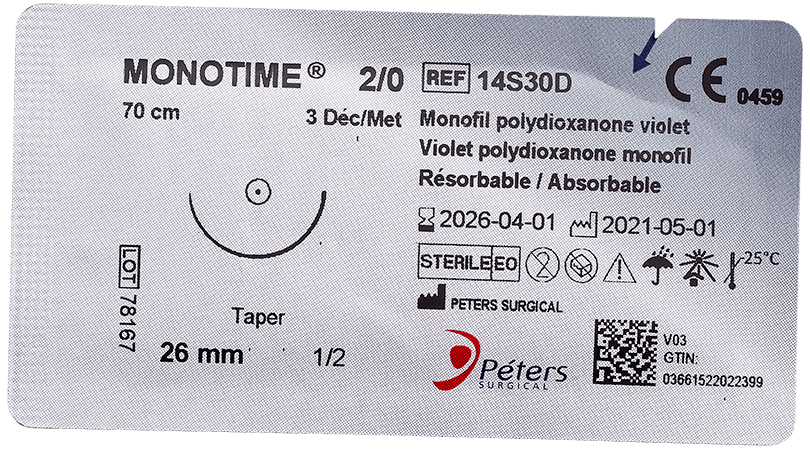
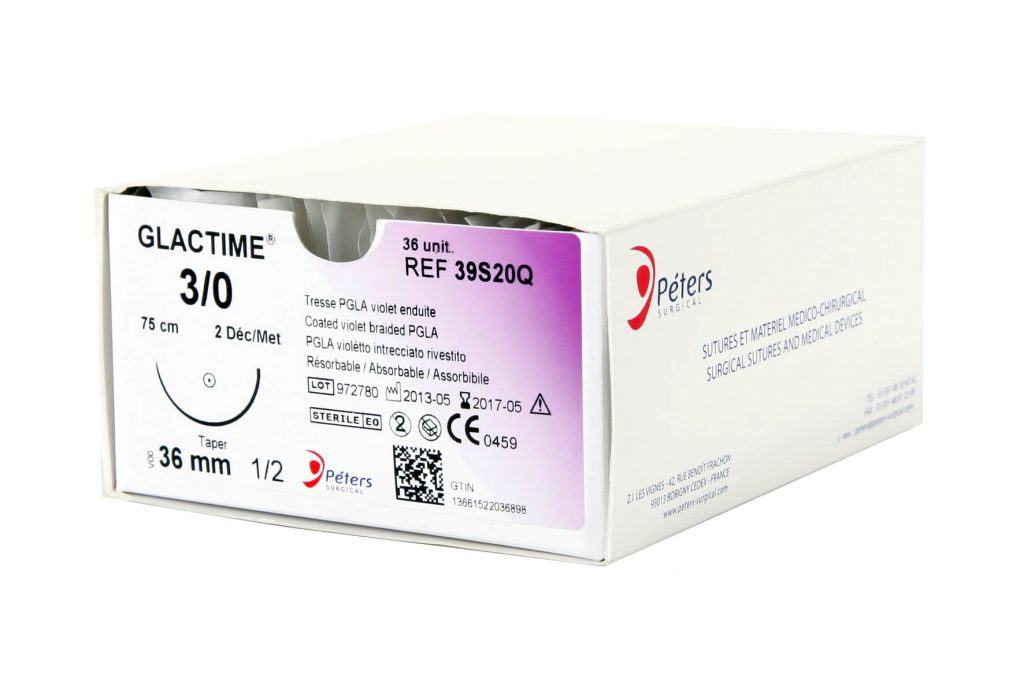
The major advantage of absorbable sutures is the absence of foreign bodies in living tissue after a few months, without any additional intervention.
Technically, absorbable sutures must have sufficient strength to hold the wound until the tissue has healed sufficiently. Then the absorbable sutures will lose all of their strength and be eliminated by the body’s metabolism or their knot falls outside the body (if it is surface use).
Absorbable sutures characteristics
- Time for complete loss of strength retention (zero retention): At the end of which the suture fulfills its role of maintaining the edges of a wound (the strength of the thread is close to zero).
- Total absorption : The time required for complete degradation and complete elimination of the suture from the body.