Back to Articles
13 November 2025
Creating anastomoses is a crucial step in coronary artery bypass grafting (CABG), serving as the means by which blood flow is redirected from the aorta to the coronary arteries, bypassing those that are narrowed or blocked. This article explores the techniques and considerations involved in the anastomosis process, focusing on the various methods used to ensure optimal graft placement and patency. It also examines the significance of certain sutures and devices used in this step and their contribution to the overall success of the surgical procedure.
As a reminder, an anastomosis is a connection or junction between two blood vessels or other tubular structures, such as nerves or intestines. In coronary bypass surgery, it refers to the site where a graft (such as a vein or artery) is attached to a coronary artery to restore blood flow to the heart.1
Several revascularization strategies exist for coronary artery bypass grafting (CABG), aiming to restore blood flow to the heart by bypassing obstructed coronary arteries. These include bypass with extracorporeal circulation (CPB), Off-Pump CABG, and Minimally Invasive Direct Coronary Artery Bypass (MIDCAB). 2
The choice of technique may vary depending on the surgeon’s preference.
This article will cover the Y-graft strategy, where both internal thoracic arteries are used to create a graft forming a “Y” configuration. This technique allows multiple coronary arteries to be revascularized simultaneously, optimizing blood flow to the heart. Generally, the internal thoracic arteries (LITA and RITA) are preferred due to their excellent blood perfusion and longevity.
When using the on-pump technique (i.e. under CPB), the surgeon employs an Intrack® aortic clamp for open surgery or a flexible Cygnet® clamp for minimally invasive procedures.
These devices temporarily halt blood flow from the aorta to the heart, allowing the creation of anastomoses under CPB.
As a reminder, the on-pump technique diverts blood flow from the heart and lungs to an external circuit, enabling cardiac immobility during surgery. This is made possible by the heart-lung machine, which substitutes for the heart and lungs during the procedure.
Aortic clamp stability is crucial during CPB (sufficient clamping force), while ensuring respect for aortic tissue integrity.
Intrack® inserts are compulsory for securely grasping the aortic adventitia and maintaining clamp placement without excessive occlusive force.
Protected clamps are valued by surgeons to avoid aortic trauma, particularly in cases of calcified aortas or fragile tissues, as seen in patients with aortic diseases or connective tissue disorders like Marfan syndrome.
Single-use Novaclip® Yellow Angled N10113 Bulldogs are useful for temporarily occluding source grafts during anastomosis, avoiding trauma to the vascular walls, particularly the endothelium.
If trauma is detected on the endothelium, it could negatively affect the graft’s longevity due to the potential development of lesions over time.
Single-use Bulldogs ensure consistent mechanical force across procedures and enhance patient safety through atraumatic jaws.
In a Y-graft strategy, the Novaclip® Yellow Angled N10113 Bulldog occludes the source graft, which is the left internal thoracic artery (LITA), while constructing the end-to-side anastomosis with the right internal thoracic artery (RITA).
Precise control of LITA is essential to ensure the success of the anastomosis and optimal blood flow to the heart.
In a Y-graft strategy, an end-to-side anastomosis is necessary.
Different types of anastomoses exist, including the end-to-side anastomosis. Here, the graft is connected to the coronary artery so that the graft’s end aligns with the lateral wall of the artery.
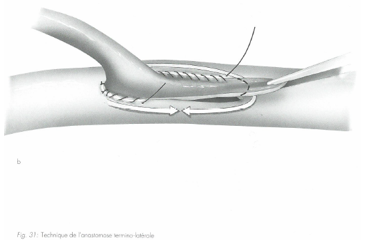
Source : « Techniques de base en chirurgie vasculaire », IMPRA Vascular Educational Services
Corolene® 8/0 (polypropylene monofilament) sutures are often used for such anastomoses. These sutures are chosen for their glide capacity, needle deformation resistance, and penetration capability in delicate vascular structures.
A suture breakage could require the surgeon to redo the anastomosis, resulting in loss of myocardial ischemia time during clamping.
Non-resorbable monofilament sutures, such as Corolene® (polypropylene monofilament) and Premio® (PVDF), are preferred for their unique properties. Polypropylene, an inert material, ensures optimal vascular anastomosis impermeability without triggering inflammation or thrombosis risk.
Its flexibility also allows the initial knots to stay in place without slipping. The benefits of polypropylene sutures include superior or equivalent resistance to competitors, a slightly thinner diameter, and excellent gliding properties, eliminating the need to wet the suture before use.
Finally, Corolene® (polypropylene monofilament) sutures’ absence of shape memory facilitates anastomosis in complex surgical conditions.
A test is performed to verify anastomosis functionality by removing the single-use Novaclip® Yellow Angled N10113 Bulldog. Additional sutures may be necessary to perfect hemostasis at the anastomosis site. Therefore, the number of sutures required in a given operation may vary even with an equal number of distal anastomoses to be performed.
The anastomosis’s impermeability depends not only on the precision of the surgical technique but also on the suture’s strength and elasticity.
The same considerations apply to distal anastomoses on the coronary arteries, which are themselves delicate structures.
The most common distal anastomosis is the side-to-side anastomosis, where the graft’s heel and tip align with the coronary artery’s heel and tip. Once the anastomosis is completed, the graft is parallel to the coronary artery.
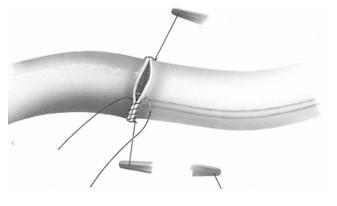
Source : « Techniques de base en chirurgie vasculaire », IMPRA Vascular Educational Services
The number and location of distal anastomoses will depend on the anatomical and pathological characteristics of each case.
For distal anastomosis between the graft (vein or artery) and the coronary arteries, continuous sutures are generally used.
The surgeon will adjust the diameter of the Corolene® monofilament suture depending on the anastomosis site and the graft used. Small sutures suitable for fragile coronary artery structures are preferred.
The suture’s gliding properties, little to no shape memory, and ease of handling in a restricted surgical field are essential to minimize tissue tear risk. This incident may lead to complex reconstructions, increased operation time and myocardial risks, and potentially suboptimal flow within the anastomosis.
Additionally, the needle must be atraumatic for delicate structures (diameter, penetration properties, and tip deformation resistance), including the graft’s intimal layer.
Good penetration is essential as calcified plaques are often encountered in coronary arteries, which may need to be circumvented or penetrated to perform the anastomosis at the desired site.
Yellow Angled Novaclip® Bulldog Clamps N10113 will also be positioned and removed repeatedly as needed.”
A side-to-side anastomosis is first performed between the LITA and the LAD at segment 2.
This first coronary target (LAD segment 2) is reached by epicardial dissection. Epicardial veins are controlled using titanium clips (4.7 mm, small-medium-lilac) to ensure hemostasis and separation.
The LITA is controlled with a Novaclip® Yellow Angled N10113 Bulldog, which will be removed at the end of this first anastomosis to verify its impermeability.
An end-to-side anastomosis is then performed between the LITA and the LAD at segment 3.
A significant stenosis exists between LAD segments 2 and 3.
When using a venous graft, as in this case with the LITA, a polypropylene monofilament suture Corolene® 7-0 or 6-0 may be used, depending on the surgeon’s preferences.
Proximal anastomoses on the ascending aorta may or may not be necessary, depending on the revascularization strategy chosen by the surgeon.
For proximal anastomosis of the venous graft (e.g., the saphenous vein) on the ascending aorta, polypropylene monofilament Corolene® sutures (5/0 or 6/0) are appropriate. They are similar to those used for distal anastomoses but with larger diameters. Their absence of shape memory facilitates surgical techniques, such as the parachute technique, for precise and tension-free insertion.
A final distal anastomosis may be performed: the RITA (right internal thoracic artery) graft will be used to revascularize an obtuse marginal branch via an end-to-side anastomosis.
Once all anastomoses are completed, the cardiac cavity is debulked, and the surgeon gradually removes the single-use Novaclip® Yellow Angled N10113 Bulldogs and the Cygnet® Aortic cross clamp to test for leaks.
Some surgeons prefer to perform the proximal end-to-side anastomosis of the venous graft on the ascending aorta AFTER removing the clamp and Bulldogs to ensure optimal hemostasis.
If the surgeon opts for this strategy, it can be performed with or without extracorporeal circulation, depending on the surgeon’s preferred technique.
To perform an anastomosis without extracorporeal circulation (beating-heart technique), it is necessary to create a blood-free space where the graft will be positioned. Two options are available:
The advantage of this beating-heart technique is the reduction of myocardial ischemia time.
In conclusion, creating anastomoses is a crucial step in CABG that directly impacts the success of the procedure. The choice of graft type, anastomosis technique, and suture material are all important factors that influence graft patency and overall outcomes. As surgical techniques and technologies continue to evolve, the potential for improved outcomes in CABG procedures increases.
NOVACLIP®
INDICATIONS:
The NOVACLIP® Surgical Spring Clip is indicated for use in peripheral vascular, cardiovascular and general surgery. NOVACLIP® provides occlusion of atherosclerotic vessels or normal vessels and may be used over indwelling catheters. NOVACLIP® may be used for temporary occlusion of the autogenous saphenous vein during coronary bypass surgery. NOVACLIP® may also be used as a suture tag.
Class IIa Medical Device- CE 2797 – Manufacturer: Peters Surgical.
Read instructions carefully before use.
COROLENE®
INDICATIONS:
COROLENE® sutures are intended for use in general soft tissue approximation and/or ligation, including use in cardiovascular and vascular surgery, in ophthalmic surgery, in plastic surgery and in neurological surgery. COROLENE® sutures can be used for laparoscopic surgery and abdominal aorta surgery.
Class III Medical Device- CE 0459 – Manufacturer: Peters Surgical.
Read instructions carefully before use.
PREMIO®
INDICATIONS : PREMIO® sutures are intended for use in general soft tissue approximation and/or ligation, including use in cardiovascular, vascular and neurological surgery.
Class III Medical Device – CE 0459 – Manufacturer: Peters Surgical.
Read instructions carefully before use.
INTRACK® SURGICAL CLAMP
INDICATIONS : Vascular clamping : Suitable for veins and arteries – Performs efficiently on either diseased or normal vessels – Provides occlusion of atherosclerotic vessels without excessive closing forces – Minimizes intimal damage and fragmentation of atherosclerotic material – Can be clamped over indwelling catheters – Gastrointestinal clamping : Replaces bulky, rubber shod clamps – Cushion-design of inserts enables occlusion without crushing the bowel.
Class Ir Medical Device – Manufacturer :Vitalitec International, Inc. d/b/a Peters Surgical USA.
Read instructions carefully before use.
INTRACK® Insert
INDICATIONS : Do not resterilize and/or reuse the INTRACK® inserts. Resterilization and/or reuse may compromise the integrity of the device. This may lead to potential risks of failure of the device to perform as intended and/or cross-contamination associated with using inadequately cleaned and sterilized devices. Proper disposal of contaminated devices is required. Proper disposal of contaminated devices is required, including disposal of reusable clamps that are no longer acceptable for use. • It is possible to inadvertently pass a needle through the jaw insert, thereby attaching the insert to the suture line. Care must be taken to avoid pinching gloved finger between the insert and the clamp channel. INTRACK® Atraumatic removable insert, must be used exclusively with INTRACK® and Cygnet® surgical clamps
Class IIa Medical Device – CE 2797 – Manufacturer :Vitalitec International, Inc. d/b/a Peters Surgical USA.
Read instructions carefully before use.
Single Use Proximal Anastomosis Assist Device ENCLOSE® II
INDICATIONS : The Enclose® II device is intended for use by cardiac surgeons during on-pump or offpump coronary artery bypass grafting (CABG) procedures in place of partial occlusion clamps in ascending aortas free of atheromatous disease. The intended purpose of the Enclose® II device is to atraumatically create hemostasis for CABG procedures without partial occlusion clamping.
Class IIa Medical Device – CE 2797 – Manufacturer :Vitalitec International, Inc. d/b/a Peters Surgical USA.
Read instructions carefully before use.
SOURCES :
1 « Réalisation de l’anastomose en Y entre les 2 artères mammaires internes »,Learncabg Chirurgie Cardiaque, Service de chirurgie thoracique et cardio-vasculaire du CHU Besançon
www.chirurgie-cardiaque-besancon.org
2 « Nos techniques : Chirurgie coronaire », Dernière mise à jour le 12/08/2024, Service de chirurgie cardiaque CHUV (Vaud, Suisse)
Pontage sans circulation extra-corporelle – Service de chirurgie cardiaque – CHUV